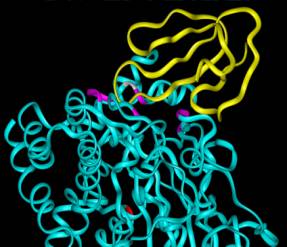
 Crystal structure of fasciculin (yellow) binding to the peripheral site of acetylcholinesterase (cyan).
Crystal structure of fasciculin (yellow) binding to the peripheral site of acetylcholinesterase (cyan).
Protein-protein interactions are important processes involved in everything from signal transduction, to protease function, to protein folding, to the formation of multimolecular protein assemblies in vivo. My research focuses on two broad questions: 1) What fundamental elements are important in determining the affinity and specificity of protein-protein interactions? and, 2) How can we interfere with or mimic these protein-protein interactions in a therapeutically significant way? We approach these general questions by focusing on two distinct enzymes.
The first is the aspartyl protease β-secretase whose activity results in the formation of the amyloid beta (Aβ) peptide. This short peptide has been shown in several studies to be intricately involved in the pathogenesis of Alzheimer’s Disease (AD) and cerebroamyloid angiopathy (CAA) resulting in higher incidence of stroke. β-secretase is one of two key proteases whose activity results in the production of this Ab peptide from the amyloid precursor protein (APP). According to the amyloid hypothesis of AD and CAA, Ab aggregation is an early and causal event along the disease pathway leading to these diseases. We will characterize the subsite specificities and kinetics of proteolysis and then apply this understanding to design inhibitors that bind with high affinity and selectivity.
| Our second area of research involves the study of acetylcholinesterase (AChE). Fasciculins (Fas) are picomolar affinity snake venom neurotoxins that inhibit AChE. Their mode of inhibition is unique in that they induce an allosteric effect in addition to blocking the active site. Protein-protein contacts between one loop of Fas and an AChE loop that forms one wall of the active site are likely involved in transmitting this allosteric effect. An understanding of this allosteric effect at the molecular level will allow us to design compounds that will block the action of neurotoxic AChE inhibitors known as organophosphates. Both of these projects will involve molecular biology, spectroscopy, electrophoresis, chromatography, cell biology, molecular modeling, and kinetic analyses. |
|
Recent Publications